CART-Wheel
Project title
Centre for Analysis of Rare Tumours
Investigators
Prof Clare Scott (Principal Investigator), Prof Peter Gibbs, Dr Jayesh Desai
Sites
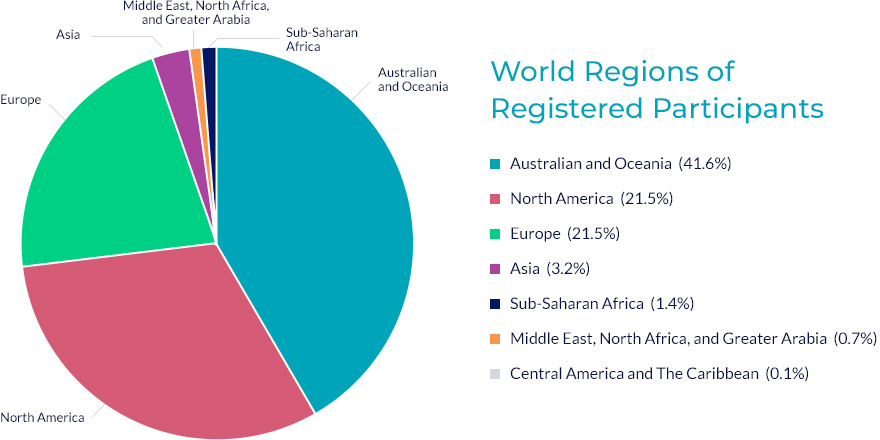
Available online to all rare cancer patients in Australia and overseas
A major roadblock to rare cancer research is the lack of data. It is difficult for one single hospital to gather enough data on any one type of rare tumour to conduct statistically meaningful research. Patients are more likely to die if they are diagnosed with a rare cancer than a common cancer, but despite this urgency, the share of the research funding pie is a small slice when compared with more common cancers.
With the participation of consumers and the expertise of BioGrid Australia, CART-Wheel.org was developed to address some of the barriers to rare cancer research.
CART-Wheel was born as a tool which allows information to be collected from patients anywhere in the world. Patients are able to enter their data into an ethically-approved and privacy-protected questionnaire, reached via the CART-Wheel website. The questionnaire gathers detailed information on tumour type, treatment received, genetic testing and aspects of family history. Data is de-identified and stored on the secure platform supplied by BioGrid.

One of the great advantages of working with BioGrid on the development of the CART-Wheel questionnaire is that we’ve been able to modify and enhance the questions over time.

“We’ve been able to add questions which cover psychosocial aspects of a rare cancer diagnosis and also quality of life. These are very important areas to look at, and quite under-researched.”
Patients with any of the more than 500 types of rare tumours can supply their medical data to CART-Wheel. Patients with rare subtypes of common cancers or an inherited rare tumour predisposition are also welcome to participate. They can provide consent for their data to be used for research purposes via an online click-button consent form.
Patients can also indicate their interest in being introduced to further ethically-approved research projects involving analysis of their tissue, such as the Walter and Eliza Hall Institute of Medical Research Stafford-Fox Rare Cancer Program, which has a remote consent option for Australians consented to CART-Wheel.
To gain access to this valuable data, rare cancer researchers apply via the standard BioGrid data access application. Requests are reviewed by an independent Expert Scientific Review Panel overseen by Melbourne Health Human Research Ethics Committee and assessed on scientific merit and feasibility before access to de-identified data is granted.

WhiMSICAL Principal Investigators Dr Ibrahim Tohidi-Esfahani (L) and Prof Judith Trotman (C) From the Concord Cancer Centre in NSW and Co-investigator Andrew Warden (R) from the Australian Consumer Group, WMozzies.
Researchers at Sydney’s Concord Cancer Centre put their case forward to access data from patients with Waldenström's macroglobulinemia, or WM for short, a rare type of non-Hodgkin lymphoma. They were granted access and initiated the WhiMSICAL project, with the support of consumer advocate groups in Australia and the United States. Additional questions were added to the CART-Wheel questionnaire to gather a greater depth of data from the WM participants.
To date, the WhiMSICAL team has analysed information from close to 400 WM patients across the world have consented to CART-Wheel and presented their findings at international meetings. Of particular interest to the group is gaining a real-world understanding of what treatment regimens are in use, and what is proving effective. These data is critical to ongoing clinical trials and to gain approval and funding for novel treatment protocols.
Download WhiMSICAL poster
Learn about our consumer partners Rare Cancers Australia
To find out more about the CART-Wheel rare cancer database, visit CART-Wheel.org
Professor Clare Scott is Professor of Gynaecological Cancer at the University of Melbourne, Laboratory Head at Walter and Eliza Hall Institute of Medical Research and Medical Oncologist, Peter MacCallum Cancer Centre, Royal Melbourne and Royal Women's Hospitals.